Medical Disclaimer: This article provides educational information about Ozempic (semaglutide) side effects. Always consult with your healthcare provider before starting, stopping, or modifying any medication regimen. This content is reviewed by licensed pharmacists (PharmD) but does not replace professional medical advice.
TL;DR – Key Takeaways
- Most common side effects: Nausea affects up to 20% of patients, diarrhea occurs in approximately 8-14%, and constipation in 3-7%
- Side effect timeline: Most gastrointestinal symptoms peak during weeks 1-4 and improve significantly by week 12
- Serious but rare risks: Pancreatitis occurs in approximately 0.3 cases per 100 patient-years, gastroparesis (stomach paralysis), and thyroid tumor warnings
- Dose-related pattern: Slow dose titration reduces gastrointestinal side effects by approximately 70% by week 8
- When to seek emergency care: Severe abdominal pain, persistent vomiting, difficulty breathing, or neck swelling require immediate medical attention
- Discontinuation: Symptoms typically improve within weeks of stopping Ozempic, though some patients report persistent issues
Ozempic Side Effects: What You Need to Know
Ozempic (semaglutide) is an FDA-approved medication for managing type 2 diabetes that helps lower blood sugar levels and protect kidney function. Understanding potential side effects helps patients make informed decisions and recognize when medical attention is needed.
The most common adverse reactions reported in clinical trials include nausea, vomiting, diarrhea, abdominal pain, and constipation, occurring in ≥5% of patients. While these gastrointestinal effects are typically manageable, Ozempic can also cause serious complications requiring immediate medical evaluation.
This comprehensive guide covers all documented Ozempic side effects, management strategies, and critical warning signs based on clinical trial data and FDA safety information.
Most Common Ozempic Side Effects
Gastrointestinal Effects
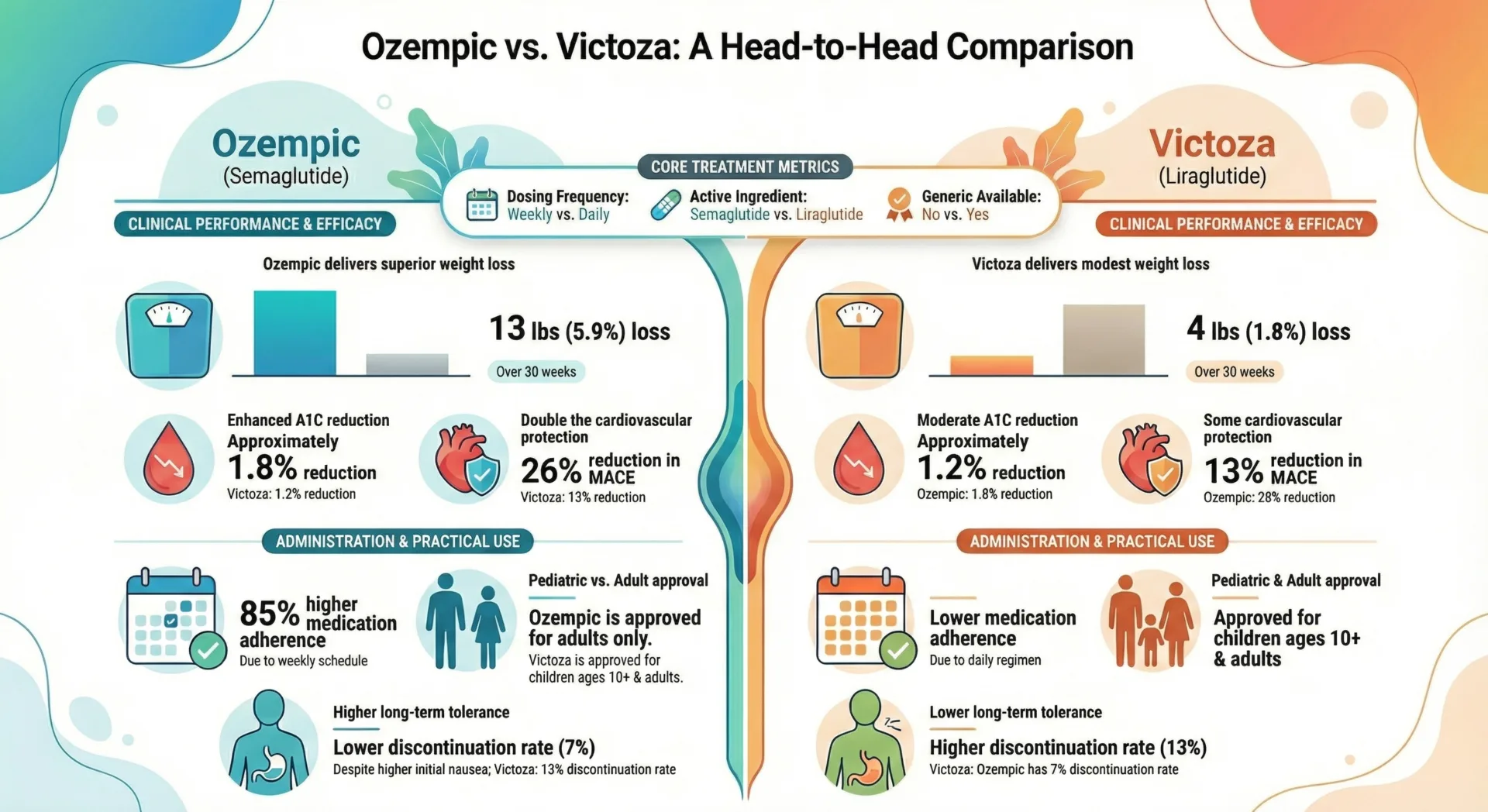
In placebo-controlled trials, gastrointestinal adverse reactions occurred more frequently among patients receiving Ozempic than placebo (15.3% placebo, 32.7% Ozempic 0.5 mg, 36.4% Ozempic 1 mg).
The specific incidence rates from clinical studies include:
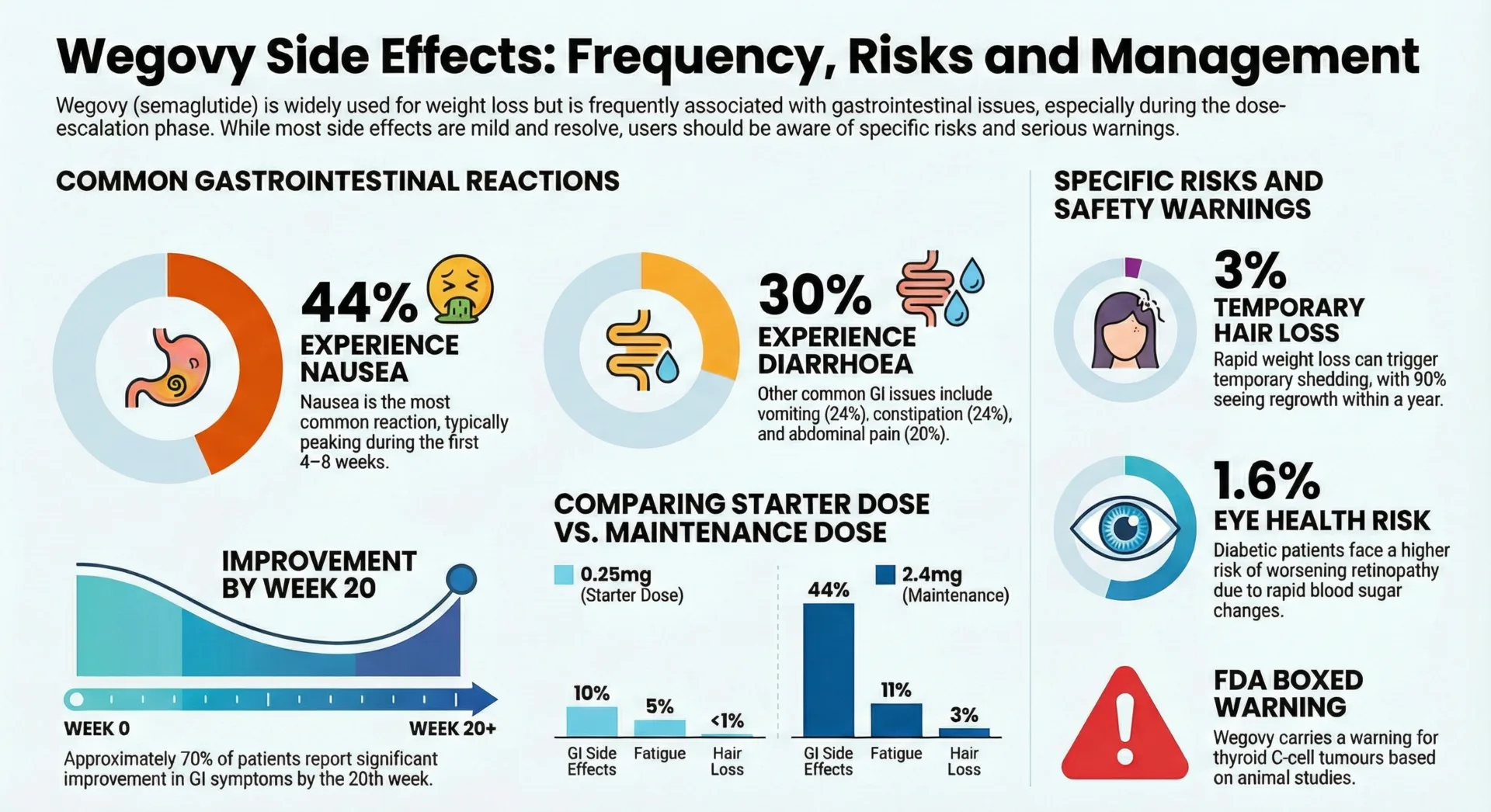
- Nausea: 15.8% of patients on 0.5 mg and 20.3% on 1 mg experienced nausea compared to 6.1% of placebo group. Nausea is typically mild to moderate and improves as your body adjusts.
- Diarrhea: Occurring in about 8% to 14% of patients, diarrhea is typically mild to moderate with frequent, loose, or watery stools.
- Vomiting: Affects approximately 5-9% of patients, often coinciding with nausea episodes.
- Constipation: Occurs in 3% to 7% of patients experiencing infrequent or difficult bowel movements.
- Abdominal Pain: Mild to moderate stomach discomfort or cramping occurs in 5% to 7% of patients.
The majority of reports of nausea, vomiting, and/or diarrhea occurred during dose escalation, emphasizing the importance of gradual dose increases.
First 4 Weeks: What to Expect and How to Manage
The initial month on Ozempic typically involves the most noticeable side effects. Proper dose titration can reduce gastrointestinal effects by approximately 70% by week 8.
Management strategies for early side effects:
- Dietary modifications: Start with smaller, more frequent meals rather than three large meals. Avoid high-fat, greasy, or spicy foods that can worsen nausea. Focus on bland, easily digestible options like crackers, toast, rice, and bananas.
- Hydration and electrolytes: Drink water consistently throughout the day. Consider electrolyte solutions if experiencing vomiting or diarrhea to prevent dehydration.
- Natural remedies: Ginger tea or ginger supplements may help reduce nausea. Probiotics can support digestive health during the adjustment period.
- Timing considerations: Some patients find taking Ozempic before bedtime reduces daytime nausea symptoms.
- Additional common effects: About 2% of participants reported gastroesophageal reflux disease (GERD) at lower doses, along with heartburn and acid reflux. Avoiding lying down for 2-3 hours after eating can help minimize these symptoms.
How Long Do Side Effects Last?
Understanding the typical timeline for Ozempic side effects helps set realistic expectations:
Week 1-4 (Peak intensity)
Side effects are most pronounced during the initial dose and first dose escalation. Nausea, vomiting, and diarrhea are most common when first starting Ozempic but decrease over time in the majority of patients.
Week 5-12 (Improvement phase)
Approximately 70% of gastrointestinal side effects resolve or significantly improve during this period as your body adapts to the medication.
Week 12+ (Stabilization)
Most patients experience substantial relief, with 90% of initial side effects resolved by month 4. In one clinical study, 83% of people taking Ozempic for 2 years developed gastrointestinal symptoms, but none developed gastroparesis.
Individual response varies based on dosage, individual tolerance, and adherence to gradual dose escalation. More patients receiving Ozempic 0.5 mg (3.1%) and 1 mg (3.8%) discontinued treatment due to gastrointestinal adverse reactions than placebo (0.4%).
If side effects persist beyond 12 weeks or worsen over time, consult your healthcare provider about potential dose adjustments or alternative treatments.
Serious Side Effects Requiring Emergency Care
1. Pancreatitis Warning
Pancreatitis (inflammation of the pancreas) is a rare but potentially life-threatening complication. In glycemic control trials, acute pancreatitis was confirmed in 7 Ozempic-treated patients (0.3 cases per 100 patient-years) versus 3 comparator-treated patients (0.2 cases per 100 patient-years).
Critical warning signs requiring immediate emergency room evaluation:
- Severe abdominal pain that radiates to the back
- Pain that doesn’t improve or worsens over time
- Pain accompanied by nausea and vomiting
- Fever along with abdominal discomfort
- Inability to eat or keep food down
Stop using Ozempic and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting.
2. Vision and Thyroid Issues
- Diabetic Retinopathy
In a 2-year trial involving patients with type 2 diabetes and high cardiovascular risk, more events of diabetic retinopathy complications occurred in patients treated with Ozempic (3.0%) compared to placebo (1.8%). The risk is higher among patients with existing diabetic retinopathy.
Patients should report any vision changes immediately, including blurry vision, dark spots, difficulty seeing at night, or sudden vision loss. Annual comprehensive eye exams are recommended for all Ozempic patients.
- Thyroid Tumors
In studies with rodents, Ozempic and medicines that work like Ozempic caused thyroid tumors, including thyroid cancer. While thyroid cancer incidence was notably low in human studies, with isolated cases constituting less than 1% within study groups, the FDA requires a boxed warning.
Tell your healthcare provider if you get a lump or swelling in your neck, hoarseness, trouble swallowing, or shortness of breath as these may be symptoms of thyroid cancer. Ozempic is contraindicated in patients with a personal or family history of medullary thyroid carcinoma (MTC) or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
Starting vs Stopping Ozempic Side Effects
Women-Specific Effects
Female patients may experience unique side effects:
- Hair thinning: Approximately 5-10% of patients experience temporary hair loss, medically known as telogen effluvium. This is typically related to rapid weight loss rather than direct medication effects and usually resolves as weight stabilizes.
- Menstrual changes: Some women report changes in menstrual cycle regularity, though reported rates are 3-5%. These changes are often temporary and related to weight loss.
- Pregnancy considerations: You should stop using Ozempic 2 months before you plan to become pregnant due to the medication’s long washout period.
Stopping Ozempic: What to Expect
Discontinuing Ozempic involves specific considerations:
- Appetite rebound: The most common effect when stopping Ozempic is the return of appetite. The medication’s appetite-suppressing effects diminish as it leaves your system.
- Weight regain: Studies suggest 50-100% of lost weight may return over 6-12 months after discontinuation without continued lifestyle modifications. This reflects the medication’s mechanism of action rather than a withdrawal symptom.
- No withdrawal syndrome: Unlike some medications, Ozempic does not cause traditional withdrawal symptoms. If a dose is missed, administer as soon as possible within 5 days after the missed dose. If more than 5 days have passed, skip the missed dose and resume the regular schedule.
- Symptom resolution: In one case study, a woman taking semaglutide who experienced stomach paralysis saw her symptoms significantly improve and nausea completely resolve within a month of stopping.
Injection Site and Missed Dose Effects
Injection location considerations
Some patients report that thigh injections may cause slightly milder nausea compared to stomach injections, potentially due to slower absorption rates. However, it is acceptable to inject Ozempic in the abdomen, thigh, or upper arm, and patients should rotate sites to prevent lipodystrophy.
Injection site reactions occur in less than 1% of patients during clinical studies, with potential redness, swelling, or pain at the injection site.
Missed dose protocol
If a dose is missed, administer within 5 days after the missed dose. If more than 5 days have passed, skip the missed dose and administer the next dose on the regularly scheduled day. Missing doses generally does not cause significant side effects but may affect blood sugar control.
Additional symptoms during initial weeks
- Headaches: Approximately 20% of patients experience headaches during weeks 1-4. These typically resolve with adequate hydration and usually don’t require treatment.
- Dry mouth: About 10% report dry mouth during the first month. Staying well-hydrated and using sugar-free gum or lozenges can help.
- Fatigue and dizziness: More than 0.4% of patients report these symptoms, which may be related to changes in eating patterns or blood sugar levels.
Long-Term and Serious Gastrointestinal Complications
Gastroparesis (Stomach Paralysis)
Gastroparesis is a serious condition where stomach emptying is severely delayed. Up to 1 in 20 new users of Ozempic and other GLP-1 medications may develop stomach paralysis, though a study analyzing FDA safety data from 2018 to early 2022 identified 48 cases of impaired stomach emptying among 5,442 reports of semaglutide-related gastrointestinal issues.
How Ozempic affects digestion: Ozempic works by slowing stomach emptying, which results in prolonged fullness and controlled appetite. In some patients, this slowing becomes excessive.
Gastroparesis symptoms include:
- Severe nausea and vomiting lasting more than an hour
- Feeling full after only a few bites of food
- Severe bloating and abdominal distension
- Unpredictable blood sugar fluctuations
- Unintentional weight loss beyond expected amounts
Risk factors: People with diabetes are at higher risk for intestinal blockage due to diabetic neuropathy, which can impair digestive function and compound Ozempic’s effects.
Diagnosis and prognosis: The gold-standard test for gastroparesis is a gastric emptying study. For many patients, symptoms improve or resolve after discontinuing Ozempic, often within weeks to months. However, some cases report persistent symptoms, and around 1% of patients taking Ozempic have a stomach paralysis diagnosis.
Bowel Obstruction and Ileus
In September 2023, the FDA updated Ozempic’s label to warn about ileus, a potentially life-threatening bowel obstruction. GLP-1 agonists were associated with a 4.22 times higher risk of bowel obstruction compared to bupropion-naltrexone.
Symptoms of bowel obstruction requiring emergency care:
- Severe cramping and abdominal pain
- Complete inability to pass gas or stool
- Severe bloating with rigid, distended abdomen
- Persistent vomiting, especially of bile or fecal matter
- No bowel movements for several days with worsening symptoms
These complications can lead to tissue death if untreated and may require surgical intervention.
Kidney Problems
There have been postmarketing reports of acute kidney injury and worsening of chronic renal failure, which may sometimes require hemodialysis, in patients treated with GLP-1 receptor agonists.
The primary risk factors include:
- Severe vomiting and diarrhea leading to dehydration
- Pre-existing kidney disease
- Inadequate fluid intake during gastrointestinal symptoms
Monitor renal function when initiating or escalating doses of Ozempic in patients reporting severe adverse gastrointestinal reactions.
Warning signs requiring medical attention:
- Decreased urine output or dark-colored urine
- Swelling in legs, ankles, or feet
- Unusual fatigue or confusion
- Persistent nausea with inability to maintain hydration
Gallbladder Problems
In placebo-controlled trials, cholelithiasis (gallstones) was reported in 1.5% and 0.4% of patients treated with Ozempic 0.5 mg and 1 mg respectively, while cholelithiasis was not reported in placebo-treated patients.
Symptoms of gallbladder issues requiring emergency evaluation:
- Severe pain in the upper right abdomen, especially after eating
- Pain radiating to the right shoulder or back
- Fever with chills
- Jaundice (yellowing of skin or eyes)
- Clay-colored stools or dark urine
Allergic Reactions and Hypersensitivity
Ozempic may cause serious allergic reactions, including anaphylaxis, which require immediate medical attention.
Symptoms of serious allergic reactions:
- Difficulty breathing or shortness of breath
- Swelling of face, lips, tongue, or throat
- Severe rash, itching, or hives
- Rapid heartbeat or dizziness
- Feeling like you’re going to pass out
Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Ozempic. If you experience any signs of an allergic reaction, stop using Ozempic and seek emergency medical care immediately.
Blood Sugar Concerns
Ozempic does not typically cause low blood sugar (hypoglycemia) on its own, but your risk increases if you use Ozempic with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin.
Signs and symptoms of low blood sugar include:
- Dizziness or lightheadedness
- Blurred vision
- Anxiety, irritability, or mood changes
- Sweating and shakiness
- Slurred speech or confusion
- Hunger and weakness
- Fast heartbeat and feeling jittery
- Headache and drowsiness
Patients using Ozempic with insulin or sulfonylureas may require dose adjustments of these medications to reduce hypoglycemia risk. Always carry glucose tablets or a quick source of sugar, and inform family members about hypoglycemia symptoms and treatment.
Cardiovascular Effects
In placebo-controlled trials, Ozempic 0.5 mg and 1 mg resulted in a mean increase in heart rate of 2 to 3 beats per minute. While this increase is generally not clinically significant, patients with pre-existing heart conditions should be monitored.
Ozempic has shown cardiovascular benefits in clinical trials. The estimated hazard ratio for time to first major adverse cardiovascular event was 0.74 (95% CI: 0.58, 0.95), demonstrating a reduction in heart attack, stroke, and cardiovascular death risk.
Other Notable Side Effects
Decreased appetite and weight loss
Decreased appetite was reported in 4% to 8% of patients taking Ozempic. While often a desired effect for diabetes management, excessive weight loss or inability to maintain adequate nutrition requires medical evaluation.
“Ozempic face” and loose skin
Rapid weight loss can lead to facial sagging or hollowing and loose skin on other body parts. This cosmetic change results from volume loss rather than direct medication effects. The extent of skin changes depends on age, skin elasticity, and amount of weight lost.
Digestive issues
Gas (burping, flatulence) and indigestion occur, though these are less common than nausea and vomiting. About 3.5% of participants reported dyspepsia (indigestion) and fewer than 2% reported flatulence.
Mood and behavioral changes
Some patients report experiencing agitation, irritability, or abnormal behaviors, though these effects are not well-documented in clinical trials. Any significant mood changes should be discussed with healthcare providers.
When to Contact Your Healthcare Provider
Routine concerns (contact within 24-48 hours):
- Persistent nausea lasting beyond the first few weeks
- Constipation not relieved by over-the-counter remedies
- Mild injection site reactions
- Questions about dose timing or administration
Urgent concerns (contact same day):
- Severe or worsening abdominal pain
- Persistent vomiting preventing adequate hydration
- Signs of dehydration (dark urine, dizziness, decreased urination)
- Unexplained rapid weight loss
- Changes in vision
- Symptoms of low blood sugar if taking other diabetes medications
Emergencies (call 911 or go to ER):
- Severe allergic reaction symptoms
- Difficulty breathing or swallowing
- Severe abdominal pain radiating to the back
- Complete inability to keep down fluids for 24+ hours
- Chest pain or signs of heart attack
- Lump or swelling in neck with breathing difficulty
Balancing Benefits and Risks
Ozempic offers significant benefits for managing type 2 diabetes, including improved blood sugar control, cardiovascular protection, and kidney disease risk reduction. Understanding potential side effects enables patients to recognize normal adjustment symptoms versus serious complications requiring medical intervention.
Most side effects are manageable and temporary, resolving within weeks to months. However, serious complications like pancreatitis, gastroparesis, and severe allergic reactions require immediate medical attention.
Always follow your healthcare provider’s dosing instructions, report concerning symptoms promptly, and maintain regular monitoring appointments.
Sources and References
- U.S. Food and Drug Administration. (2025). OZEMPIC (semaglutide) Prescribing Information
- Sodhi, M., et al. (2023). Risk of Gastrointestinal Adverse Events Associated with GLP-1 Receptor Agonists for Weight Loss. JAMA Network
- University of British Columbia Faculty of Medicine. (2025). Weight-loss drugs linked to stomach paralysis, other serious gastrointestinal conditions
- National Institutes of Health. (2024). Assessment of Thyroid Carcinogenic Risk and Safety Profile of GLP1-RA Semaglutide
- Novo Nordisk. (2025). Ozempic Important Safety Information
Disclaimer: This information is intended for general knowledge and informational purposes only and does not constitute medical advice. Always consult with a healthcare professional for personalized guidance.
Written by the Pandameds.com Editorial Team
Our content is created by pharmacy-trained researchers and healthcare specialists and rigorously reviewed by a diverse panel of authentic experts from the pharmaceutical and healthcare fields. This collaborative review process ensures that every article meets the highest standards of medical accuracy, reliability, and relevance.
- ✅ Authored by pharmacy-trained professionals
- 🔍 Reviewed by multiple verified experts in the pharmaceutical and healthcare niche
- 💊 Based on trusted sources including FDA, Health Canada, and peer-reviewed clinical studies
- 🔄 Regularly reviewed and updated every 90 days to maintain accuracy and trustworthiness
About Pandameds.com
Pandameds.com offers a range of weight loss medications at an affordable price.
Fast, Reliable Shipping to the USA!
Affordable Prescription Meds From Canada
Join our mailing list for exclusive promos, curated health content & more.
Frequently Asked Questions
Is diarrhea a side effect of Ozempic?
Yes, diarrhea was the third most commonly reported side effect in clinical trials, with about 8% of people experiencing it while taking Ozempic. Diarrhea usually occurs during the first weeks and improves over time. Limiting high-fat foods, sugary beverages, and caffeine can help reduce symptoms.
Is constipation common with Ozempic?
Yes, constipation occurs in 3% to 7% of patients with infrequent or difficult bowel movements. It's most common during the first 28 days of starting Ozempic. Increasing fiber intake through fruits, vegetables, and whole grains, along with adequate water consumption, typically helps manage this side effect.
Does Ozempic cause pancreatitis?
Pancreatitis is rare but documented. In glycemic control trials, acute pancreatitis occurred in 0.3 cases per 100 patient-years in Ozempic-treated patients. Watch for severe abdominal pain radiating to the back. If this occurs, stop Ozempic and seek immediate emergency care.
What happens if you miss an Ozempic dose?
If a dose is missed, administer as soon as possible within 5 days after the missed dose. If more than 5 days have passed, skip the missed dose and resume the regular once-weekly dosing schedule. Missing a dose typically doesn't cause side effects but may affect blood sugar control temporarily.
Are headaches normal on Ozempic?
Yes, headaches affect approximately 20% of patients during weeks 1-4 of treatment. They usually resolve with proper hydration and rarely require treatment. If headaches are severe or persistent, consult your healthcare provider.
Is hair loss from Ozempic permanent?
No, hair thinning from Ozempic is typically temporary telogen effluvium caused by rapid weight loss rather than the medication itself. Hair growth usually resumes as weight stabilizes. The condition affects approximately 5-10% of patients and is not permanent.
What are Ozempic eye side effects?
Diabetic retinopathy complications occurred in 3.0% of Ozempic patients compared to 1.8% of placebo patients in a 2-year trial. The risk is higher among patients with existing diabetic retinopathy. All diabetic patients on Ozempic should have annual comprehensive eye exams to monitor for vision changes.
What side effects occur after stopping Ozempic?
The primary effect is appetite rebound as the medication's appetite-suppressing effects diminish. Patients may experience 50-100% weight regain over 6-12 months without continued lifestyle modifications. There is no traditional withdrawal syndrome from stopping Ozempic.
Does thigh injection reduce Ozempic side effects?
Some patients report slightly milder nausea with thigh injections compared to stomach injections, potentially due to slower absorption. However, clinical evidence for this difference is limited. Ozempic can be injected in the abdomen, thigh, or upper arm, and rotating injection sites is recommended.
What are the most common Ozempic side effects?
The most common adverse reactions reported in ≥5% of patients treated with Ozempic are nausea, vomiting, diarrhea, abdominal pain, and constipation. These gastrointestinal effects typically improve with gradual dose titration and usually resolve within 8-12 weeks.
Related Blog Posts
Call Us Today!
If you have any questions, please email our support team at [email protected] or call us toll-free at 1-888-862-1210.